
|
|||||
| Application number: | P200200472 | |
| Patent number: | 05098 | |
| PCT application number: | EP01/02055 | |
| Filing date of the application: | 22.02.2001 | |
| Publication date: | 15.12.2003 | |
| Date of the grant of the patent: | 15.12.2008 | |
| Priority date: | 24.02.2000 | |
| Estonian title : | Molsidomiini pikendatud vabanemisega oraalne galeeniline ravimvorm | |
| English title : | Oral galenical form with prolonged release of molsidomine | |
| Status: | Patent protection period expired | |
| Fees paid for the years of validity : | 20 | |
| IPC class: | A61K9/16 A61K9/20 A61K9/22 A61K31/535 A61P9/10 | |
| CPC class: | ||
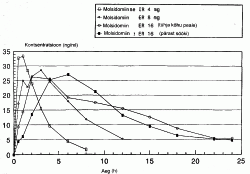
| Estonian abstract: | Käesolev leiutis puudutab molsidomiini uut pikendatud vabanemisega suukaudselt manustatavat galeenilist ravimvormi stenokardia kõigi vormide (pingutus- ja rahuolekustenokardia, ebastabiilne stenokardia) ravimiseks. Vastavalt leiutisele sisaldab uus ravimvorm terapeutiliselt tõhusa koguse molsidomiini või üht selle aktiivset metaboliiti ja selle lahustumismäär in vitro [Pharmacopée Europeénne 3. väljaandes (või U.S.P XXIV) kirjeldatud meetodi järgi spektrofotomeetriliselt mõõdetult 286 või 311 nm, kiirusel 50 p/min, keskkonnas 500 ml HCl 0,1 N ja temperatuuril 37 °C] on: 15-25 % molsidomiinist vabaneb l tunni möödudes; 20-35 % molsidomiinist vabaneb 2 tunni möödudes; 50-65 % molsidomiinist vabaneb 6 tunni möödudes; 75-95 % molsidomiinist vabaneb 12 tunni möödudes; rohkem kui 85 % molsidomiinist vabaneb 18 tunni möödudes; rohkem kui 90 % molsidomiinist vabaneb 24 tunni möödudes, molsidomiini in vivo mõõdetud tippkontsentratsioon saabub 2,5-5 tunni, soovitavalt 3-4 tunni möödumisel nimetatud ravimi sissevõtmisest ja selle väärtuseks on 25-40 ng/ml plasmast. Leiutist kasutatakse ravis.@ | |
| English abstract: | The invention concerns a novel galenical form for oral administration with prolonged release of molsidomine for treating angina attack in its various forms (exertion angina, spastic angina, mixed angina). The invention is characterised in that said novel galenical form contains a therapeutically efficient amount of molsidomine or one of its active metabolites and it has a dissolution rate in vitro [measured by spectrophotometry at 286 or 311 nm according to the European Pharmacopeia, 3rd edition (or U.S.P XXIV) at 50 t.p.m in 500 ml of a HCl 0.1 N medium, at 37 ?C] of: 15 to 25 % of molsidomine released after 1 hour, 20 to 35 % of molsidomine released after 2 hours, 50 to 65 % of molsidomine released after 6 hours, 75 to 95 % of molsidomine released after 12 hours, > 85 % of molsidomine released after 18 hours, > 90 % of molsidomine released after 24 hours, the plasma peak of molsidomine obtained in vivo occurring between 2.5 to 5 hours, preferably between 3 to 4 hours, depending on the administration of said form and exhibiting a value between 25 and 40 ng/ml of plasma. The invention has therapeutic uses. | |
| Applicant/holder: |
Therabel Pharmaceuticals Limited | |
| Inventor name: | Jozsef-Michel Geczy (BE) | |
| Drawing(s): Click on the drawing to display a larger drawing! |
|
|
| Published patent application: | Display the published patent application (Document A) | |
| Patent specification: | Display patent specification (Document B) | |
| Patent attorney: | Lembit Mitt | |
| Display: | DATA OF THE FEES | |
|
|